STAKEHOLDER INTERVIEWSPerspectives from 15 global health leaders on GHIT's catalytic role and
Japan's transformational contributions to global health R&D
DEVELOPMENT
02
Mr. Yoshihiko Hatanaka
Representative Director, President and CEO
Astellas Pharma Inc.
“Improving Access to Health is one of the most important pillars of our corporate social responsibility.”
What motivated your company to engage in global health?
We have long been interested in contributing to global health and Access to Health using our company’s technologies and capabilities. Improving Access to Health is one of the most important pillars of our corporate social responsibility (CSR). Indeed, we have made strong commitments to solve problems related to Access to Health in our Strategic Plan 2015-2017.
Our company’s former chairman, Mr. Nogimori, was appointed Vice President of the International Federation of Pharmaceutical Manufacturers & Associations in 2010. He played a major role in Astellas’ initial engagement in global health. Through his appointment, we gained greater awareness of the importance of various global health issues and countermeasures through dialogues with other global pharmaceutical companies, the World Health Organization, and governments of other nations. Since then, we have proactively considered how to utilize our capabilities in support of global health, which, as I mentioned, is a CSR priority.
Our efforts started around 2010, when infectious diseases were one of our company’s focus therapeutic areas. Astellas has various formulation technologies, so it made sense to contribute to Access to Health using those technologies. Subsequently, we began to engage in various projects, such as infectious diseases collaborations with other partners from industry, government, and academia, as well as the Pediatric Praziquantel Consortium for schistosomiasis.
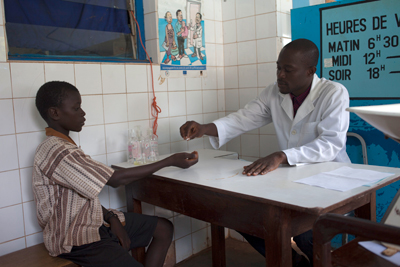
Clinical site of Phase II trial for pediatric praziquantel in Ivory Coast
What are some examples of your flagship global health activities?
An example of our engagement in Access to Health is our involvement in the Pediatric Praziquantel Consortium, an international, non-profit consortium that aims to deliver drugs with appropriate formulations to children with schistosomiasis, a Neglected Tropical Disease (NTD). The consortium was established in 2012, and besides our company, Merck KGaA, Darmstadt, Germany (Merck KGaA) and other organizations are partners. Praziquantel is a standard therapeutic drug with reliable evidence to treat schistosomiasis. The use of the drug was initially limited to adults and school-aged children. Because praziquantel pills were not suitable for infants and preschool-aged children due to their large size and strong bitter taste, there has not been any effective treatment for them. We decided to participate in the Consortium with the intention of applying our company’s expertise in formulation technologies to this problem, to help redesign the drug so that it could also be delivered to infants and preschool-aged children.

At first glance, this activity does not directly contribute to your bottom line as a company. What motivated Astellas’ management team to make this commitment?
As the story goes, in an academic conference held in Holland in 2011, one of the members of Astellas’ development division happened to be seated next to a representative of Merck KGaA, which originally developed praziquantel. The Merck KGaA had contributed to control schistosomiasis treatment with praziquantel. However, as previously mentioned, the current formulation could not be successfully used for treating infants and preschool-aged children. The Merck KGaA, representative’s passion for solving this challenge resonated with the Astellas scientist. This interaction catalyzed internal discussions about whether our formulation technologies could be used.
So why did the management support this initiative? In the end, I believe the priority given to Access to Health in our CSR was the driving factor. The pharmaceutical industry itself has long contributed to society by creating new drugs. We believe that making use of our company’s technologies and capabilities for broader unmet needs can help millions of patients who are living with and fighting diseases.
It is important to acknowledge that the pharmaceutical business requires long-term, high-risk investment in research and development (R&D). If definite commercial success is not guaranteed, even if sufficient medical needs exists, the priority for the R&D tends to decrease. The activities of the Pediatric Praziquantel Consortium themselves do not lead to short-term or direct business profits. However, the return for human health is enormous, and very valuable to us.
Additionally, there is also strategic value in the relationships with partner companies we developed through activities like the Consortium, local partnerships and networks inside developing countries, understanding of specific issues faced by developing countries, and relationships with each nation’s government. Ultimately, these activities enhance our enterprise value by earning trusts from stakeholders and help us execute on our mission. In this sense, there was absolutely no reason for the management to object to this partnership.
“We believe that making use of our company’s technologies and capabilities for broader unmet needs can help millions of patients who are living with and fighting diseases.”
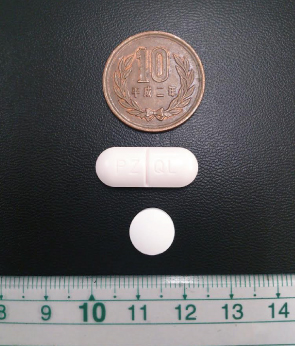
Existing tablet (top) and newly developed
pediatric formulation (bottom)
Photo Credit:Astellas Pharma Inc.
Why is developing a drug that can be administered to children particularly challenging?
When developing praziquantel formulations for use in children, we first developed mini tablets with a diameter of 2-3 mm with the intention that multiple pills could be taken based on the patient’s bodyweight. However, we learned a number of things after consulting clinical experts on the ground. Praziquantel was commonly being provided through mass drug administration in schools, and altering the number of tablets according to the child’s bodyweight was too intricate to conduct on-site. Furthermore, there is a risk of asphyxiation when administering multiple tablets to small children.
On the basis of these findings, we re-planned the formulation from scratch and developed an orally dispersible tablet using our company’s drug formulation technologies. This drug could immediately dissolve in the mouth, even without water. Through this process, we arrived at the current formulation of the tablets. By miniaturizing the tablets to be only a quarter of the size of the current formulations, as well as minimizing their bitter taste, they will be easier for small children to swallow and enable treatment with fewer tablets. Because schistosomiasis spreads in hot and humid regions, we then focused on ensuring the stability of the formulation.
In our company, we hold the business philosophy that “Contribute toward improving the health of people around the world through the provision of innovative and reliable pharmaceutical products.” We are conscious that the work of Astellas is turning innovative science into value for patients. With this mindset as a foundation, a culture has emerged wherein our employees habitually consider whether the company’s technologies and strengths can be exploited to solve societal issues. For example, drug lag is a constant problem for pediatric therapeutic agents, and our company’s Pharmaceutical Research & Technology Labs. have initiated independent research into pediatric formulations to solve this problem.
I believe that these activities demonstrate our employees’ aspirations to take advantage of Astellas’ capabilities, and their own abilities, for society’s benefit.

Astellas is a founding partner of the GHIT Fund. What features of the GHIT model or approach were most appealing to your management team when the institution was first conceived?
As I previously mentioned, we have developed a strategy of engaging in global health issues, including R&D. Actually, at the same time that this strategy was first being implemented, we were also involved in early discussions about GHIT’s establishment. GHIT’s objectives of supporting the R&D of new drugs, vaccines, and diagnostics for controlling infectious diseases in developing countries and the commitment to solve these problems through global partnerships were very unique. At the same time, I was impressed by GHIT’s unique public-private partnership model. This particular scheme, facilitating concrete engagement and funding from the governments, private sectors, and foundations—aimed at addressing global issues that cannot be resolved by a single company alone—was truly innovative. This inspired me to ensure our participation in GHIT. Importantly, we did not receive any objections from our stakeholders including shareholders.
Obviously, companies should invest in profitable projects. On the other hand, I believe that also engaging in activities that make a social value and fulfill social responsibility rather than just pursuing commercial success not only enhances trust from our stakeholders but also increases the overall value of the company.

Has Astellas’ engagement in global health R&D delivered any new insights or lessons to the company about the product development process?
First, through our involvement in the Pediatric Praziquantel Consortium, we learned a profound lesson about understanding the complexities on the ground when it comes to delivering drugs in the developing world. For example, we had to be creative with the administration of the drug while simultaneously reducing production costs, simplifying production techniques, and ensuring stability in the hot and humid conditions of tropical regions. These challenges are not typically major factors for product development for the developed world settings in which we usually operate. This was an exceedingly valuable experience for our company.
Second, by engaging in drug development for NTDs, we learned new lessons about partnerships with external research organizations, the governments of developing countries, and other stakeholders. Open innovation is a critical: we have seen how collaboration among multiple interested parties across sectors produces something of greater value.
Third, as I previously mentioned, this work has fostered a new culture within the company. Employees are engaging in these activities of their own accord, which means a great deal to us.
“GHIT catalyzed partnerships between the Japanese pharma industry and global entities that we would not have been able to create on our own.”
How do you think the Japanese pharmaceutical industry’s overall engagement in global health R&D has evolved over the past five years?
I believe that GHIT has catalyzed a mindset shift for the Japanese pharmaceutical industry vis-à-vis global health R&D. Global health is clearly an international and cross-border issue. Our company is a member of the Japan Pharmaceutical Manufacturers Association (JPMA), which has 72 pharmaceutical companies as members, all of whom, I believe, have all experienced this mindset shift following GHIT’s establishment. More specifically, GHIT created a mechanism for these companies to take leadership roles in solving global health issues by combining their R&D expertise and capabilities in cooperation with those of governments, international organizations, and non-profits. This contribution is a direct result of GHIT’s establishment.
Next, while it is difficult for private companies to take long-term risks in the field of global health on their own, GHIT’s support appears to have greatly boosted the active engagement of the pharmaceutical industry in this arena.
Furthermore, GHIT’s global network of stakeholders is extremely valuable. GHIT catalyzed partnerships between the Japanese pharma industry and global entities that we would not have been able to create on our own. Additionally, GHIT has expanded our awareness of partners that are engaged in the same issues. This has played a major role in helping us to leverage our capabilities and technologies to solve global health issues.
Looking ahead to the next five years, what kind of initiatives will be necessary to further leverage Japanese innovation for global health?
Japan’s Ministry of Health, Labour and Welfare created the Japan Vision: Health Care 2035, which includes becoming a global health leader as a key target. JPMA also stated in its Industry Vision 2025 that it would provide innovative drugs to 8 billion people worldwide. In that respect, I believe we can deliver Japan’s innovation to patients suffering from diseases worldwide through open innovation in partnership with entities around the world.
The affiliations and positions listed in this interview are at the time of publication of the interview in 2017.

- Biography
- Yoshihiko Hatanaka
Representative Director, President and CEO
Astellas Pharma Inc.
Yoshihiko Hatanaka became Astellas’ President and CEO in June 2011. Hatanaka served as the company’s Senior Corporate Executive, Chief Financial Officer and Chief Strategy Officer from June 2009 to May 2011. He joined the marketing organization of Fujisawa Pharmaceutical Co., Ltd. in 1980 and expanded his roles and responsibilities in Japan, the US and Europe. He became Fujisawa’s Vice President of the Corporate Planning in 2003 and played a key role in the merger of Fujisawa Pharmaceutical Co., Ltd. and Yamanouchi Pharmaceutical Co., Ltd. to form Astellas Pharma Inc. in 2005. He kept the senior level position under the new company. He became Astellas’ Corporate Executive and assumed the roles of President and CEO of Astellas US LLC and Astellas Pharma US, Inc. in 2006. In this position, he led Astellas’ North American operations. He holds a bachelor’s degree in Economics from Hitotsubashi University, Tokyo, Japan.
STAKEHOLDER INTERVIEWSARCHIVES
FUNDING
-

01
Dr. Naoko YamamotoSenior Assistant Minister for Global Health,
Ministry of Health, Labour and Welfare
#
-

02
Dr. Hannah KettlerSenior Program Officer, Life Science Partnerships
Global Health Program, Office of the President
Bill & Melinda Gates Foundation
#
-

03
Prof. Stephen CaddickDirector, Innovations Division,
Wellcome Trust
#
DISCOVERY
-

01
Dr. David ReddyCEO
Medicines for Malaria Venture (MMV)
#
-

02
Mr. George NakayamaRepresentative Director,
Chairman and CEO
Daiichi Sankyo Company, Limited
#
-

03
Prof. Kiyoshi KitaProfessor Emeritus, The University of Tokyo
Professor and Dean, Nagasaki University School of Tropical Medicine and Global Health
#
DEVELOPMENT
-

01
Mr. Christophe WeberRepresentative Director, President and CEO
Takeda Pharmaceutical Company Limited
#
-

02
Mr. Yoshihiko HatanakaRepresentative Director,
President and CEO
Astellas Pharma Inc.
#
-

03
Dr. Nathalie Strub WourgaftMedical Director
Drugs for Neglected Diseases initiative (DNDi)
#
ACCESS
-

01
Dr. Jayasree K. IyerExecutive Director
Access to Medicine Foundation
#
-

02
Mr. Tetsuo KondoDirector
United Nations Development Programme (UNDP)
Representation Office in Tokyo
#
-

03
Dr. Aya YajimaTechnical Officer
Malaria, other Vectorborne and Parasitic Diseases Unit
Division of Communicable Diseases
World Health Organization Western Pacific Regional Office
#
POLICY
-

01
Dr. Mark DybulFormer Executive Director
The Global Fund to Fight AIDS,
Tuberculosis and Malaria
#
-

02
Dr. Seth BerkleyCEO
Gavi, the Vaccine Alliance
#
-

03
Hon. Prof. Keizo TakemiMember of the House of Councillors of Japan
Chairman, Special Committee on Global Health Strategy
of the Liberal Democratic Party's Policy
#

